


Halvorsen and Sigurd Skogestad, Norwegian University of Science and Technology (scroll down to: 2.2. Equilibrium flash of a multi-component liquid.Α = ( y i / x i ) ( y j / x j ) = K i / K j values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries, petrochemical and chemical plants, natural gas processing plants and other industries. Read this K-value off the chart (approximately 21.3).For a liquid mixture of two components (called a binary mixture) at a given temperature and pressure, the relative volatility is defined as Note where the line crosses the methane axis. DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and.Connect the points with a straight line.(A modified DePriester chart is attached.) Problem 4. On the right-hand vertical axis, locate and mark the point containing the temperature 60☏. of 500 kg/hour while acetone (the solvent) is supplied counter-currently in stage N.Understanding the concept behind Flash Distillation, the vapor-liquid equilibrium. 2.2.2.3 Depriester Chart for Light Hydrocarbons 2.2.3. De Priester chart values and Antoine Constant table is also attached with the. It is also one of the most important processes to learn in Mass Transfer / Separation Process Technologies as it is a fundamental unit operation. K (or DePriester) Chart (low T range) in American Engineering Units A 'unit operation' is one step in a process to convert a raw material into some useful chemical product. On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia. Flash Distillation is one of the most important Mass Transfer Operations used extensively in the Chemical industry.
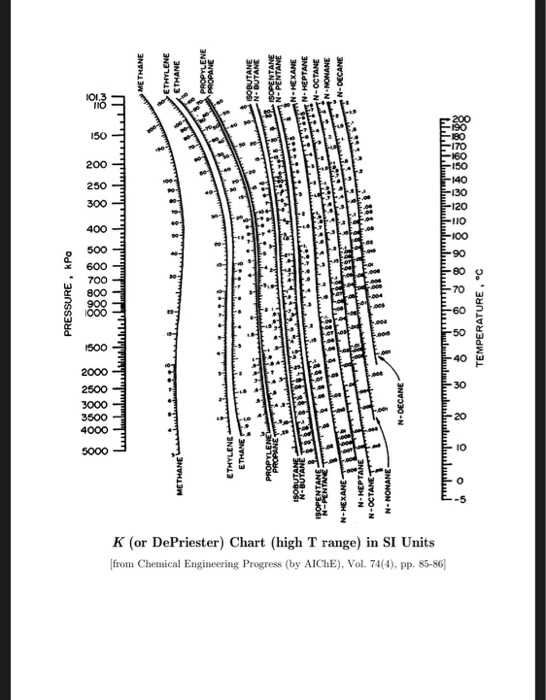
Example įor example, to find the K value of methane at 100 psia and 60 ☏. Many DePriester charts have been printed for simple hydrocarbons. "K" values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between. These nomograms have two vertical coordinates, one for pressure, and another for temperature. DePriester in an article in Chemical Engineering Progress in 1953. DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature.


 0 kommentar(er)
0 kommentar(er)
